Spark Plasma Sintering = Spark Plasma Synthesis
Topic
Solid-state reactions (SSR) are most widely used for the preparation of equilibrium (thermodynamically stable) solids (intermetallic compounds, oxides, borides, etc.), as well as for non-equilibrium (metastable) phases from the precursors (elements or / and complex compounds). SSR’s require diffusion and mass transport of the reagents. The oxidation states of the elements often change during reactions (redox reactions). Defects are a prerequisite for diffusion in solids. The problem of mass transport / diffusion acceleration in SPS is intimately connected to the kinetics of the solid state reactions and the formation of known and new phases. The previously studied of the reaction between the metals (Ti, Mo, W) – and the respective non-conductive oxides (TiO2, MoO3, WO3) demonstrated the impact of the SPS for the synthesis of the binary oxides with an intermediate oxidation state of the metals and the adaptive structures. The metal particles, as conductors of electrons, “dissolve” in the nonconductive oxide matrix by forming intermediate conductive phases. The formation of the final phase is a therefore redox diffusion-controlling reaction (Figure).
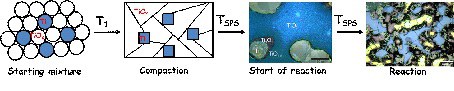
The primary goal of future investigations is using the pulsed dc current during the SPS procedure for the solid state synthesis of different phases and establishing the reaction mechanisms. The non-equilibrium conditions in SPS may allow the formation of metastable non-equilibrium compounds. Improving our understanding of these new aspects of solid state synthesis by investigating new systems during the SPS procedure is important, in particular, as various complex oxides are under investigation as promising materials for many different applications (e.g. for the thermoelectrics or batteries).
Contact person(s)
Prof. Dr. Wolfgang Tremel | Dr. Igor Veremchuk |
|---|---|
Johannes-Gutenberg-Universität Mainz Institute for Inorganic and Analytical Chemistry | Max-Planck-Institut for Chemcial Physics of Solids |
Duesbergweg 10-14 55099 Mainz | Möthnitzer Str. 40 01187 Dresden |
Tel: (+49) 6131 39 25135 | Tel: (+49) 351 4646 1118 |
Fax: (+49) 6131 39 25605 | Tel: (+49) 351 4646 4002 |
Proj.-Nr. TR 210/36-1 | Proj.-Nr. VE 948/2-1 |

